- The FDA could authorize a coronavirus vaccine as early as the end of this week.
- The move would be a pivotal moment in the pandemic, as public health officials say the U.S. is likely to face its worst public health crisis in history this winter.
- An FDA advisory group is scheduled to convene on Thursday to review Pfizer's vaccine.
The Food and Drug Administration could authorize a coronavirus vaccine as early as the end of this week. The move would be a pivotal moment in the pandemic, as public health officials say the U.S. is likely to face its worst public health crisis in history this winter.
The FDA is scheduled to convene a meeting of its Vaccines and Related Biological Products Advisory Committee, known as VRBPAC, on Thursday to review Pfizer's COVID-19 vaccine with German drugmaker BioNTech for emergency use authorization.
James Hildreth, a member of the committee, told NBC's "Weekend Today" on Saturday that an authorization could come as early as Friday.
"If the FDA commissioner decides to issue approval, the EUA, on that day when the vote is taken, as early as Friday of next week we could see vaccinations happening across the country," he said.
Emergency use authorization means the FDA will allow some adults to receive the vaccine as the agency continues to evaluate data. It isn't the same as a full approval, which can typically take months. The FDA granted emergency clearance for Gilead Sciences' remdesivir in May before giving full approval in late October.
Two days before the meeting, the FDA is expected to release a roughly 100-page document evaluating the companies' clinical trial data, said Dr. Paul Offit, a voting member of the advisory committee, offering a glimpse into the agency's view of the vaccine.
Money Report
"The public will see everything that we see," said Offit, who is also the director of the Vaccine Education Center at Children's Hospital of Philadelphia.
If the meeting Thursday goes well and the advisory committee formally votes to OK the vaccine, the FDA could announce its authorization "within days," Health and Human Services Secretary Alex Azar told ABC News' "This Week" on Sunday. "But it's going to go according to FDA's gold-standard process, and I'm going to make sure it does," he added.
The vaccine could not come at a more crucial time. Hospitals across the U.S. already have a higher load of COVID patients than ever before, and the country's outbreak is primed to set even more grim records this week. Dr. Deborah Birx, the White House coronavirus response coordinator, warned on Sunday that the escalating coronavirus surge is likely to be the most trying event in U.S. history.
"This is not just the worst public health event. This is the worst event that this country will face, not just from a public health side," she told NBC's "Meet the Press," echoing comments made by Centers for Disease Control and Prevention Director Dr. Robert Redfield on Wednesday.

"This fall/winter surge is combining everything that we saw in the spring with everything we saw in the summer — plus the fall surge going into a winter surge. I think that's why Dr. Redfield made this absolute appeal to the American people," she added.
The authorization would also mark a record-breaking time frame for a process that normally takes about a decade. The fastest-ever vaccine development, for mumps, took more than four years and was licensed in 1967. Last week, the United Kingdom authorized Pfizer's vaccine for emergency use, becoming the first country to do so.
Pfizer submitted its COVID vaccine data to the FDA on Nov. 20. The company said a final analysis of its phase three clinical trial, with more than 43,000 participants, found the vaccine was 95% effective in preventing COVID, was safe and appeared to fend off severe disease.
The FDA's review of the vaccine was expected to take a few weeks as the agency looked over every aspect of the data submitted in the application, including reviewing all safety information "to make sure there are no cracks" and everything is "solid," Offit said.
"When you talk about a 44,000-person trial, that's a lot of clinical data," Offit told CNBC in a recent interview.
U.S. officials say they will distribute the vaccine within 24 hours of authorization. Initial doses of the vaccine will be limited as manufacturing ramps up, with top U.S. health officials predicting it will take months to immunize everyone who wants to be vaccinated against COVID in the U.S. The federal government has deals lined up with several drugmakers to buy some of their first doses.
Drugmakers and states are gearing up to distribute the vaccine starting in about a week. The Federal Aviation Administration said it supported the "first mass air shipment" of vaccines late last month. United Airlines carried Pfizer's COVID vaccine from Brussels to Chicago O'Hare International Airport, people familiar with the matter told CNBC.
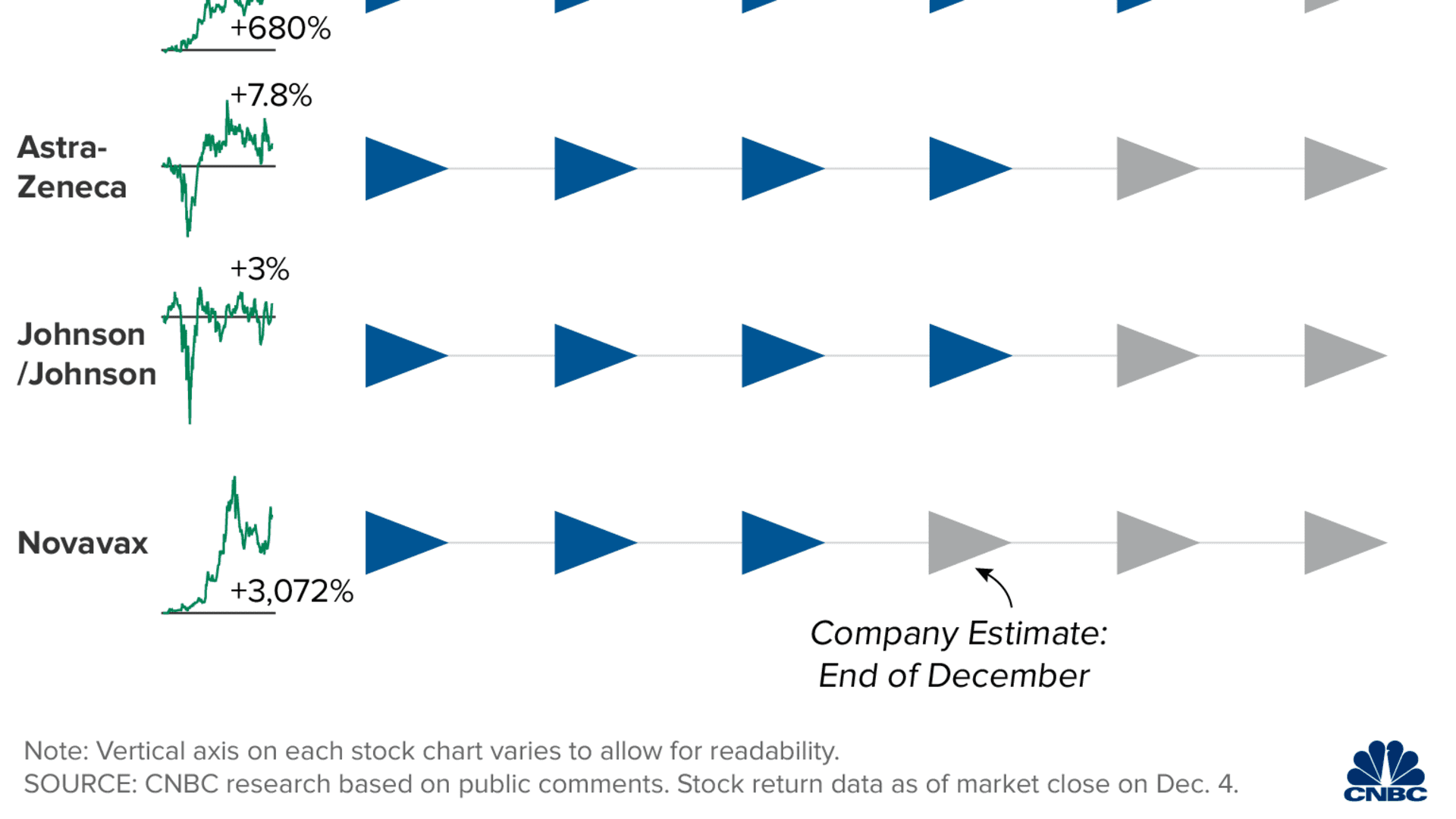
The vaccine will likely be distributed in phases. A CDC panel on Tuesday voted 13-1 to give health-care workers and long-term care facility residents in the U.S. the first doses of coronavirus vaccine once it's cleared for public use. There are roughly 21 million health-care workers and 3 million long-term care facility residents in the United States, according to the CDC.
U.S. officials say they should be able to distribute enough coronavirus vaccine doses to immunize 100 million people by the end of February, about a third of the U.S. population.
Former Presidents Barack Obama, George W. Bush and Bill Clinton said last week that they may take a coronavirus vaccine publicly, as polls suggest many Americans are skeptical about getting vaccinated. People of color, who have been disproportionately affected by the virus, in particular, appear to be less eager to take it, according to a recent Gallup poll.
Asked about skepticism of COVID vaccines on Monday, President Donald Trump's coronavirus vaccine czar, Dr. Moncef Slaoui, told CNBC that he is "very concerned" about it.
"Over the last several months, the politicization, the context under which the vaccine development has taken place has exacerbated these feelings," Slaoui, who is leading the Trump administration's vaccine program Operation Warp Speed, said in a "Squawk Box" interview. "We plan to be 100% transparent, to have as many independent experts look into all the data as can be."
He urged Americans to "keep your minds open."
"Listen to the experts that you trust. Once you hear the data in full, then make up your mind," he said.






